Calcium-Carbonate and Silica-Scaling on Reverse Osmosis Membranes
Introduction
Membrane processes are nowadays mostly used in the production of water of the highest quality. With reverse osmosis it is possible to remove all sorts of "impurities" very effectively, such as small solid particles, microorganisms, dissolved and colloidal substances.
Therefore reverse osmosis is a valuable process for the production of potable and high-purity water. A reverse osmosis system is significantly influenced by the raw water quality, the operation pressure, the temperature and the recovery rate. Due to the concentration of retained substances on the brine side, one of the main problems in membrane systems, the so-called scaling can occur.
Scaling happens when the concentration of a compound exceeds its solubility limit resulting in the formation of deposits on the membrane surface.
This occurs preferably in the last elements of a membrane system. Typical scaling forming compounds are calcium carbonate and silica. The scaling layer first reduces the permeate flux and the permeate quality, later it will block the total membrane element.
Scope of our Research Work
In this project the effect of different water compositions and different anti-scalants on the scale formation is investigated for calcium carbonate and silica.
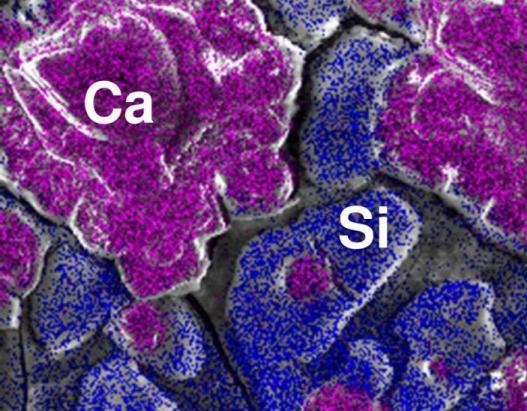
The image shows a SEM image of a RO-membrane scaled by silica and calcium carbonate. The element distribution of Ca and Si is shown in different colours. It is clearly to be seen that silica and calcite are present in two spatially separated structures on the membrane.